What Best Describes the Transition From Gas to Liquid
For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient kinetic energy. The transition of the solid phase to the gaseous phase without passing the intermediate liquid phase is known as sublimation.
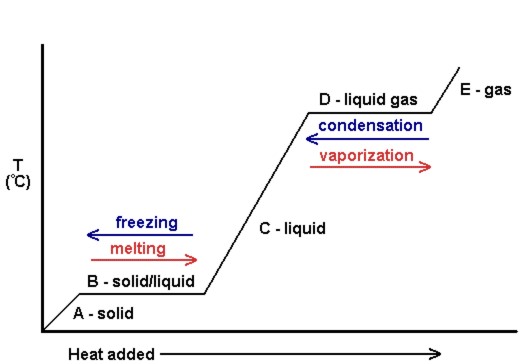
Phase Transitions Melting Boiling And Subliming Introductory Chemistry 1st Canadian Edition
17 Which best describes the particles of a liquid compared to those of a gas a from SCIENCE 20033400 at Everglades High School.

. Sublimation is the transition of a substance directly from the solid to the gas state without passing through the liquid state. In chemistry thermodynamics and many other related fields phase transitions or phase changes are the physical processes of transition between a state of a medium identified by some parameters and another one with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter.
This process requires an output of energy to. Fusion which can be as simple as melting butter in a pan is something that. Energy must be removed because particles in liquid move more slowly.
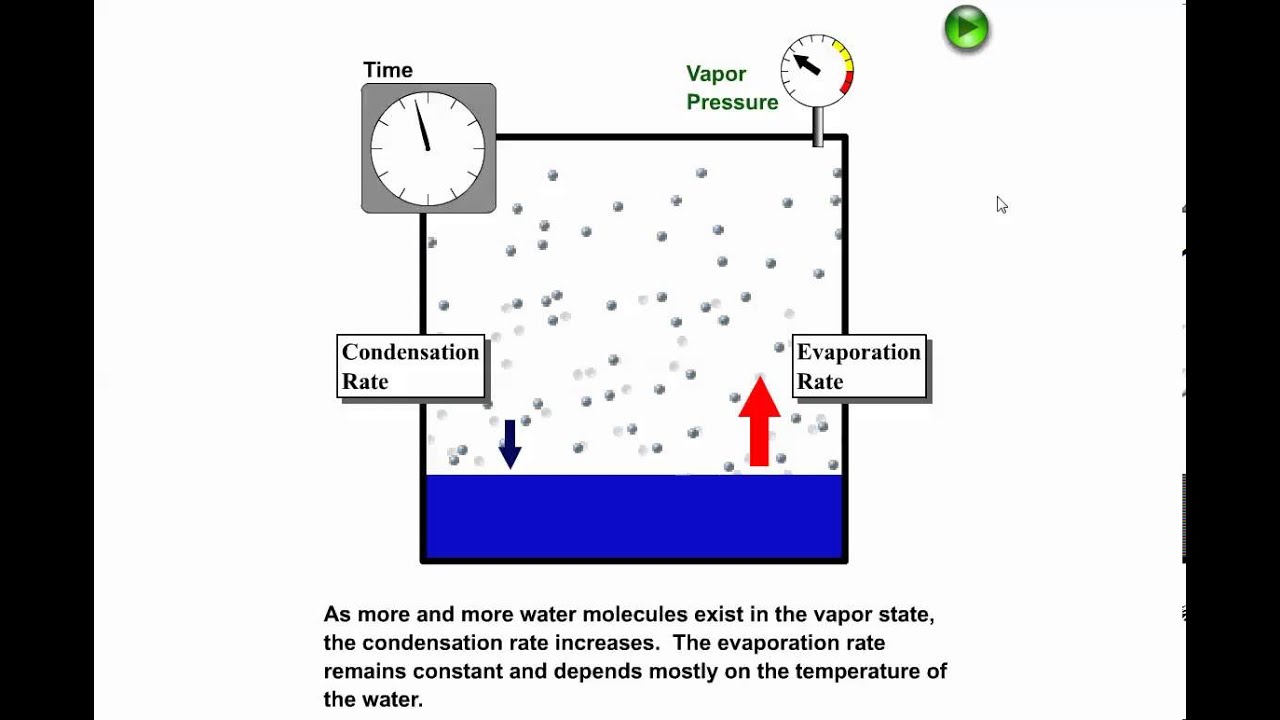
Which statement best describes the compressibility of a gas. H_2Og rarr H_2Ol Delta The process as written is exothermic and of course the condensation reaction can be used to drive a steam engine. Similarly expanding a liquid beyond the co-existence curve results in an meta-stable superheated liquid.
The process of transferring from gas to liquid is termed as condensation. Which best describes the transition from gas to liquid. Energy must be removed because particles in liquid move more slowly.
It requires the release of energy from the gas molecules to condense them as liquid. Energy must be removed because particles in liquid move more slowly. Which best describes the transition from gas to liquid.
There are three states of matter. Evaporation is a phase transition from the liquid phase to the gas phase that occurs at temperatures below the boiling point at a given pressure. Fusion is just one of approximately different states that matter can phase through.
When a solid turns into a liquid it is called fusion more commonly referred to as melting. In which phase transition do molecules move directly from a state involving vibration of particles in a fixed position to a state involving random movement of high. Energy must be removed because particles in liquid move more slowly.
This process is called deposition. For molecules of a liquid to evaporate they must be located near the surface be moving in the proper direction and have sufficient. When a material moves from one state of matter into another it is called a phase transition.
Energy is neither added nor removed in this phase transition. Gas molecules inherit large amount of energy in order to move fast as the particles of gas moves faster compared to liquid. The process by which a substance changes from the liquid phase to the gaseous phase is known as evaporation.
Both a and b d. The process by which a solid changes to a gas c. -When a gas transitions from a gaseous to a solid state bypassing the liquid state this is known as deposition.
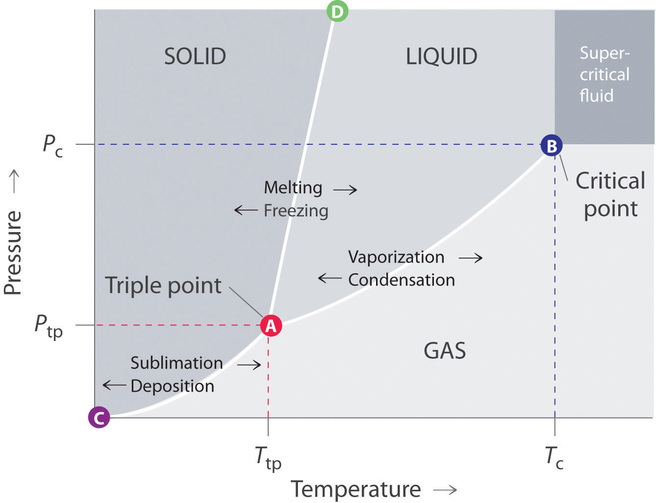
Which term best describes liquid behavior under pressure. 512 The Clausius-Clapeyron Equation We can also choose to plot the liquid-gas phase dia-Tc liquid gas p critical point T Figure 40. Energy must be removed because particles in liquid move more slowly.
3 on a question. Condensation describes the phase transition. Energy must be added because particles in liquid move more quickly.
Solid liquid and gas as well as. Vaporization or evaporation is the process by which molecules undergo a spontaneous transition from a liquid phase to a gas phase. The process by which a substance changes from the gaseous phase to the liquid phase is known as condensation.
Student Name Potential Energy Rating lowest to highest Henry gas liquid solid Jasmine gas solid liquid Lucas solid gas liquid Tasha solid liquid gas Four students rank the potential. Rank the phases of matter from the one with the least kinetic energy to the. Which best describes the transition from gas to liquid.
Bend the rubber tube to form a concave mirror and place in the ripple tank. Condensation the opposite of evaporation is the change in. So the transition must remove energy.
A state of matter that consists of loose free moving particles which form the shape set by the boundaries of the container in which the liquid is in. Here the co-existence region is. Describe the process of vaporization.
Which best describes the transition from gas to liquid. Energy must be added because particles in liquid move more quickly. CEnergy is neither added nor removed in this phase transition.
Energy is neither added nor removed in this phase transition. Gram on the pT plane. Energy is neither added nor removed in this phase transition.
For the electronic transition from n 3 to n 5 in the hydrogen atom. So ans is a. Energy is either removed or added depending on the type of particles.
Increasing the pressure on a gas decreases the volume. Follow these directions and answer the questions. Examples of Gas to Solid Deposition Under certain circumstances gas can transform directly into a solid.
Liquid solid and gas. Which best describes the transition from gas to liquid. Energy is either removed or added depending on the type of particles.
Sublimation is an endothermic process that occurs at temperatures and pressures below a substances triple point in its phase diagram which corresponds to the lowest pressure at which the substance can exist as a liquidThe reverse. Describe the process of vaporization. The water level must be below the top of the hose.
Energy is either removed or added depending on the type of particles. Energy is either removed or added depending on the type of particles. Energy must be added because particles in liquid move more quickly.
Energy must be added because particles in liquid move more quickly. Condensation Gas Liquid Sirintra Pumsopa Getty Images. Of the gas to condense into the liquid.
This photo displays the process of condensation of water vapor into dew drops. Gas is higher energy state than liq. A phase transition is the transition from one state of matter to another.
Thus the extra energy need to be released before converting it. Key Takeaways Key Points. Which best describes the transition from gas to liquid.
The process by which a liquid changes to a gas b. Water vapor to ice - Water vapor transforms directly into ice without becoming a liquid a process that often occurs on. Set up the ripple tank as in previous investigations.
This happens because the motion of the individual particles. Gas rarr liquid And thus steam water vapour condenses to liquid water. Evaporation is a phase transition from the liquid phase to the gas phase that occurs at temperatures below the boiling point at a given pressure.

No comments for "What Best Describes the Transition From Gas to Liquid"
Post a Comment